Who we are

Our capabilities
We have technical expertise in injection molding, advanced industrial tools, and regulatory competence. This allows us to offer both standard and customized products to support our clients in innovation and success.
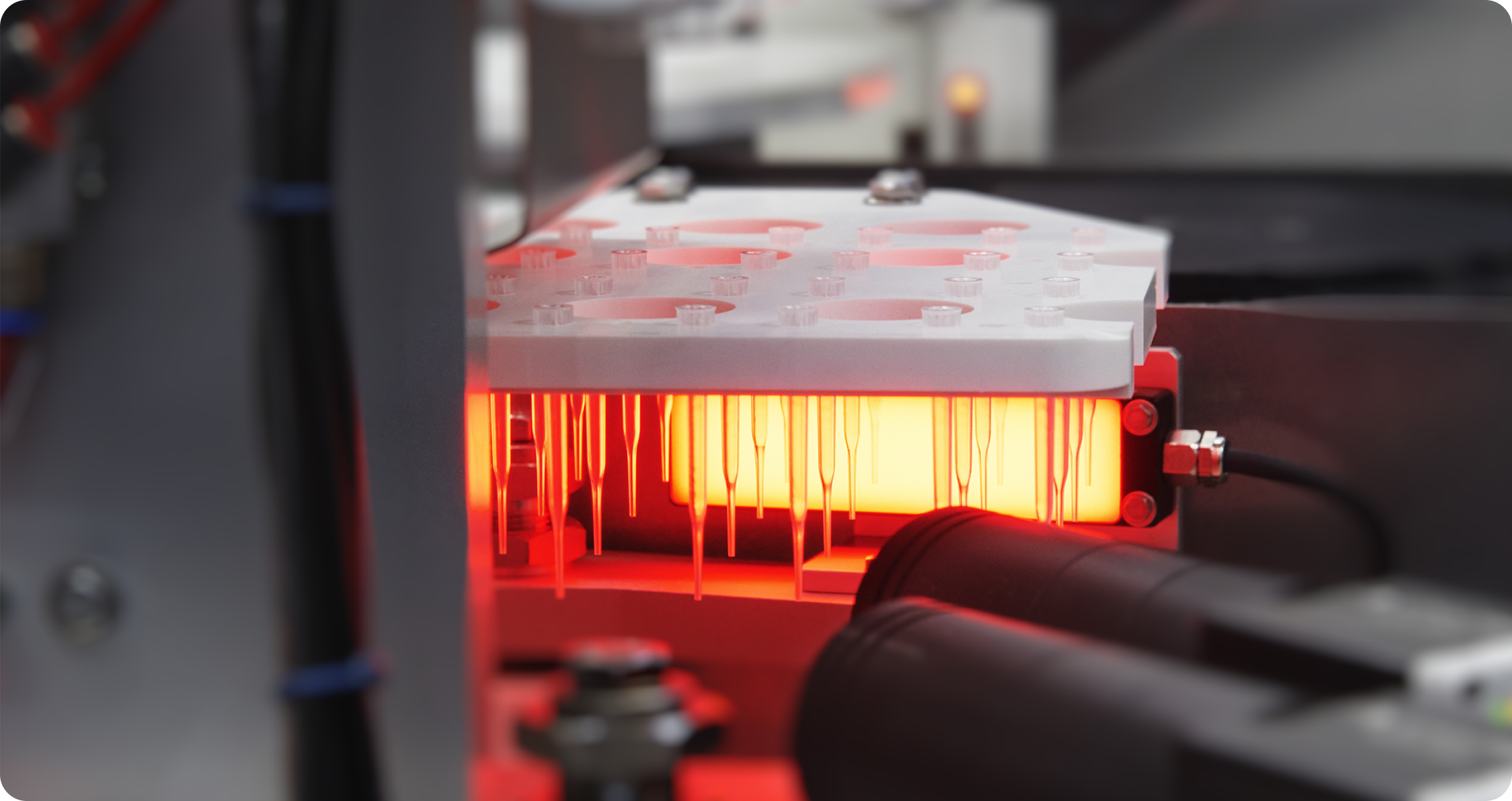
SGH Medical Pharma's primary focus is high-precision plastic injection, which is complemented by the acquisition of additional skills.
Product and process engineering
We create and develop new drug administration solutions to help you materialize your innovation goals.
Manufacturing and supply management
We are always improving our industrial and logistical infrastructure at all our production sites. This helps us anticipate your needs while reducing our environmental impact.

Regulatory compliance
From product development to commercialization, the expertise of our teams ensures compliance with all required international standards and regulations.

Our values
Innovation
We rely on the expertise in development and production to innovate and design usuable applicable medical devices, with a global technological vision and a sustainable-ethical approach.
Agility
Multisite and responsive, we are commited to maintaining this innovative mindset, no matter the circumstances and requests, with the added capacity to deliver.
Willingness
Everything is possible ! Thanks to the “doing together” approach, our teams are mobilized to go ever further, for more useful innovations, product performance and customer innovation in respect of the environment.
Strategy
Our ambition is to create a European group that specializes in drug administration, life sciences, and diagnostics.
SGH Medical Pharma aims for a turnover of 100 million euros by 2026. This will allow us to achieve the critical size sought by our clients and address the structural costs related to regulatory requirements. Our CSR policy reflects our commitment to the standards, regulations, and ethical principles we support and uphold on a daily basis.
We must promote a culture of compliance and excellence to build a strong group. To achieve this, we will integrate societal and environmental considerations into our decision-making process. We are committed to addressing the impacts of our decisions and activities on society and the environment.

Our story
-
1984
The ROVIP GROUP diversifies its business in healthcare, in a new entity to become ROVIPHARM
-
1985
Creation of STIPLASTICS
-
1995
Creation of ESKISS
-
2003
STIPLASTICS and ROVIP both get ISO 13485
-
2006
Creation of ROVIPHARM
-
2017
STIPLASTICS builds a new 10,000m² manufacturing plant in Saint-Marcellin
-
2018
Creation of SGH HEALTHCARING, reuniting STIPLASTICS, ESKISS and ROVIPHARM
Involvment of Merieux Equity Partners and GIMV
-
2019
Shift towards IVD with first strategic agreements as a Contract Manufacturer
IQ DOSE gets a Pharmapack Award
-
2021
New ISO 8-7, DNA RNA free, 100% automated cleanroom on the ROVIPHARM site
-
2022
First filtered pipette tips production batches
SGH Healthcaring becomes SGH MEDICAL PHARMA
-
2023
New cleanroom
-
2024
STIPLASTICS site will become a Pharmaceutical Establishment
600m² plant extension
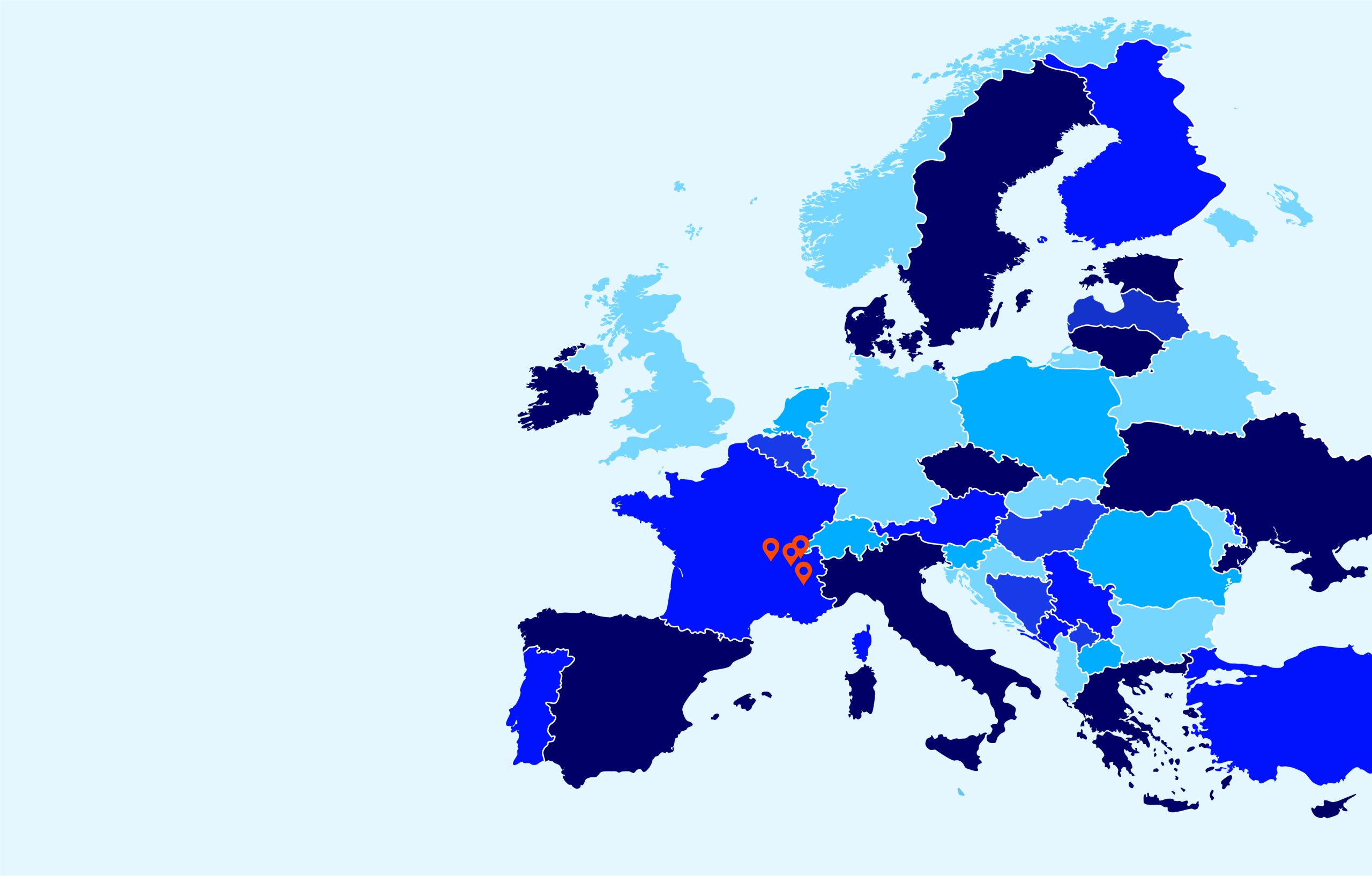
Locations
Our 250 employees at 5 sites across Europe are dedicated to helping you succeed.
Tour Oxygène
10 – 12 Boulevard Vivier Merle
69003 Lyon, France
62 chemin des Plantées
38160 Saint-Marcellin, France
Tel. : +33 4 76 38 08 44
86 route du Plan d’eau
01370 Val-Revermont, France
Tel. : +33 4 74 42 39 39
Parc Naturopôle
03800 Saint-Bonnet-de-Rochefort, France
Tel. : +33 4 70 58 85 51